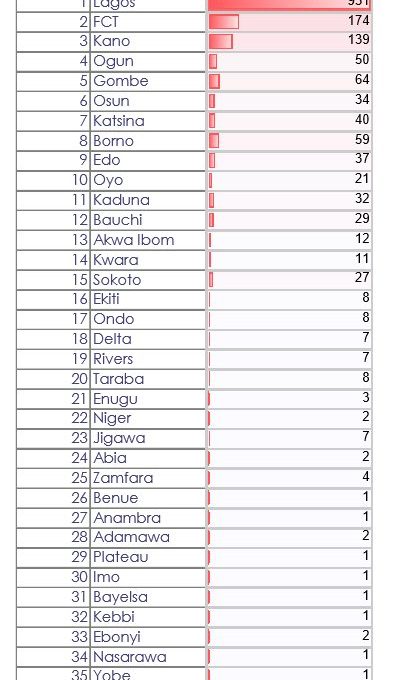
Chidi Samuel| Nigeria on Wednesday night confirmed 196 new cases of the novel coronavirus bringing the country’s toll 1728.
Even as the number of infections of the highly contagious virus continue to rise in the country and globally, there was a silver lining in the sky on Wednesday as an experimental antiviral drug, remdesivir, has been found to effective for the treatment of COVID-19 in a trial which began in February in US.
The Nigeria Centre For Disease Control made the announced the new infections in twelve states via its verified Twitter handle late on Wednesday night.
According to the health agency, Lagos, the epicentre of the disease in the country recorded 87 new infections. The other states include Kano, 24, Gombe 18, Kaduna 17, FCT 16, Katsina 10, Sokoto 8, Edo 7, Bornu 6, Yobe 1, Ebonyi 1 and Adamawa 1.
The agency further revealed that 307 of the patients have been discharged nationwide while 51 persons have died from the infection.
The Tweet read, ”196 new cases of #COVID19 reported;
87-Lagos
24-Kano
18-Gombe
17-Kaduna
16-FCT
10-Katsina
8-Sokoto
7-Edo
6-Borno
1-Yobe
1-Ebonyi
1-Adamawa
”As at 11:55pm 29th April- 1728 confirmed cases of #COVID19 reported in Nigeria”.
Discharged: 307
Deaths: 51

–Remdesivir found effective for treatment of COVID-19
Meanwhile help seem to be on the way for those stricken with the virus as an experimental antiviral drug, remdesivir, originally produced for the treatment of Ebola has been found to effective for the treatment of COVID-19.
Gilead Sciences, a biopharmaceutical company in the US, and manufacturesr of the drug made the disclosure on Wednesday, stating that data from the clinical trial was positive.
Gilead Sciences said it administered the drug on 397 severely ill COVID-19 patients and more than half of them were discharged within two weeks.
According to Aruna Subramanian, a Stanford University infectious diseases professor who led the study, the patients showed improvement after the treatment.
He said, “These data are encouraging as they indicate that patients who received a shorter, 5-day course of remdesivir experienced similar clinical improvement as patients who received a 10-day treatment course.”
According to a data cited by the US government, the experimental coronavirus treatment remdesivir has shortened the recovery time for COVID-19 patients.
“The data shows that remdesivir has a clear-cut significant positive effect in diminishing the time to recovery,” said Dr. Anthony Fauci, the government’s top infectious-disease expert, during a White House press conference on Wednesday.
A trend toward fewer deaths was seen among those on the drug, said Fauci. The study was run by National Institutes of Health [NIH] and tested remdesivir versus usual care in 1,063 hospitalized coronavirus patients around the world. Fauci said patients on the drug took 11 days on average to recover versus 15 days for the others.
“It is a very important proof of concept because what it is proving is that a drug can block this virus,” Fauci added. “The mortality rate trended towards being better in the sense of less deaths in the remdesivir group – 8 percent versus 11 percent in the placebo group. It has not reached statistical significance but the data needs to be further analyzed.”
The company said it will report the outcome of the first 600 patients in the clinical trial by the end of May.
It added that the clinical trial will also be done in the UK, China, France, Germany, Hong Kong, Italy, Japan Korea, the Netherlands, Singapore, Spain, Sweden, Switzerland and Taiwan.
Remdesivir was originally developed by the company as a potential treatment for Ebola, but it failed.
As at April 29th, 2020, #COVID19 confirmed cases have been reported in 34 states and the Federal Capital Territory



Leave a Reply