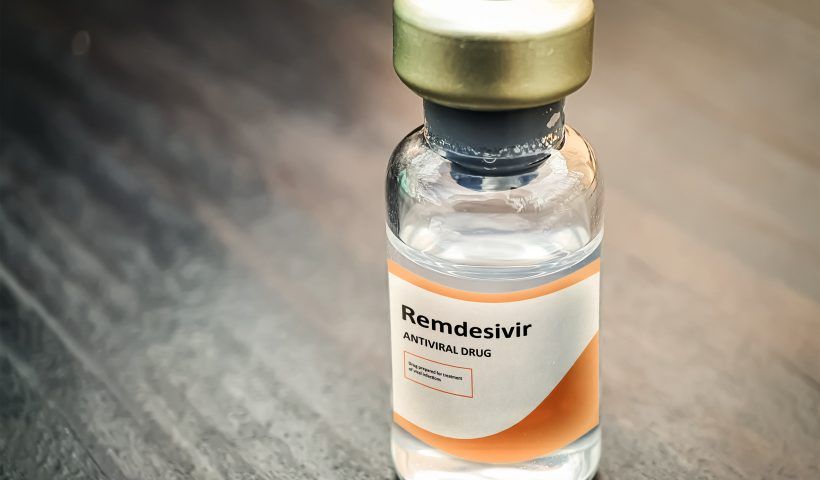
The U.S. Food and Drug Administration (FDA) on Friday granted authorization to Gilead Sciences Inc (GILD.O) for emergency use of its experimental antiviral drug remdesivir to treat patients with COVID-19,…
Read More US approves remdesivir for emergency treatment for COVID-19 as Nigeria exceeds 2000 cases